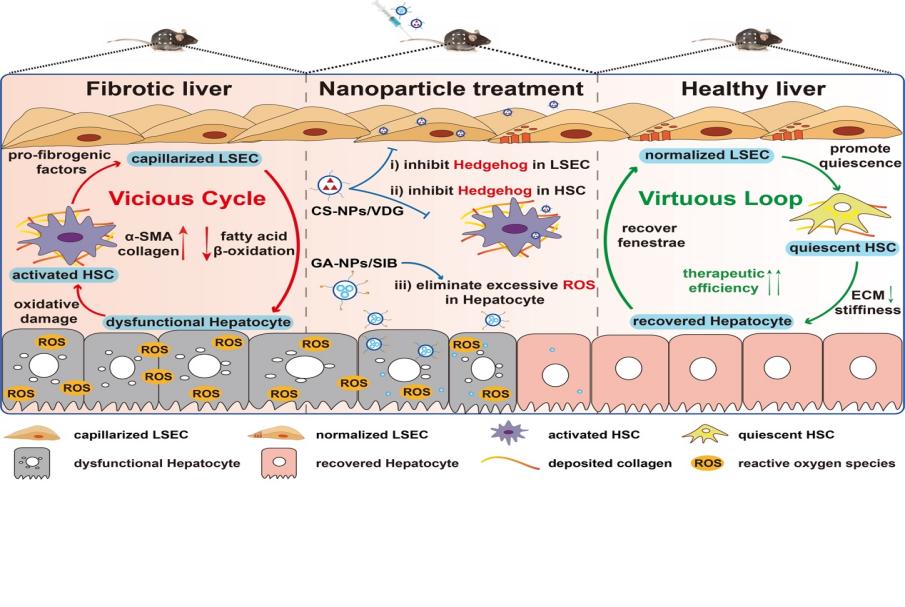
Vicious Cycle-Breaking Lipid Nanoparticles Remodeling Multicellular Crosstalk to Reverse Liver Fibrosis
Abstract
During liver fibrogenesis, the reciprocal crosstalk among capillarized liver sinusoidal endothelial cells (LSECs), activated hepatic stellate cells (HSCs), and dysfunctional hepatocytes constructs a self-amplifying vicious cycle, greatly exacerbating the disease condition and weakening therapeutic effect. Limited by the malignant cellular interactions, the previous single-cell centric treatment approaches show unsatisfactory efficacy and fail to meet clinical demand. Herein, a vicious cycle-breaking strategy is proposed to target and repair pathological cells separately to terminate the malignant progression of liver fibrosis. Chondroitin sulfate-modified and vismodegib-loaded nanoparticles (CS-NPs/VDG) are designed to efficiently normalize the fenestrae phenotype of LSECs and restore HSCs to quiescent state by inhibiting Hedgehog signaling pathway. In addition, glycyrrhetinic acid-modified and silybin-loaded nanoparticles (GA-NPs/SIB) are prepared to restore hepatocytes function by relieving oxidative stress. The results show successful interruption of vicious cycle as well as distinct fibrosis resolution in two animal models through multiregulation of the pathological cells. This work not only highlights the significance of modulating cellular crosstalk but also provides a promising avenue for developing antifibrotic regimens.
Article:https://doi.org/10.1002/adma.202311474
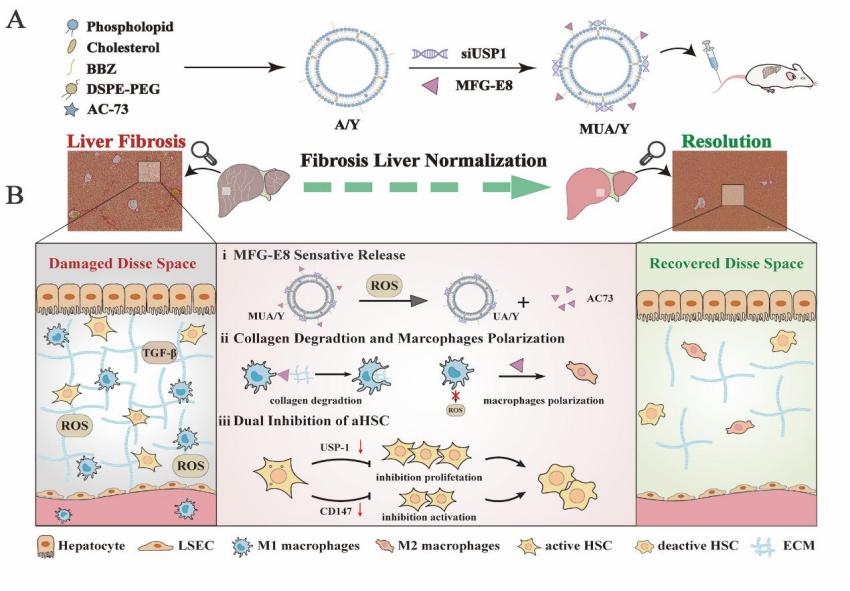
An Autologous Macrophage-Based Phenotypic Transformation-Collagen Degradation System Treating Advanced Liver Fibrosis
Abstract
In advanced liver fibrosis (LF), macrophages maintain the inflammatory environment in the liver and accelerate LF deterioration by secreting proinflammatory cytokines. However, there is still no effective strategy to regulate macrophages because of the difficulty and complexity of macrophage inflammatory phenotypic modulation and the insufficient therapeutic efficacy caused by the extracellular matrix (ECM) barrier. Here, AC73 and siUSP1 dual drug-loaded lipid nanoparticle is designed to carry milk fat globule epidermal growth factor 8 (MFG-E8) (named MUA/Y) to effectively inhibit macrophage proinflammatory signals and degrade the ECM barrier. MFG-E8 is released in response to the high reactive oxygen species (ROS) environment in LF, transforming macrophages from a proinflammatory (M1) to an anti-inflammatory (M2) phenotype and inducing macrophages to phagocytose collagen. Collagen ablation increases AC73 and siUSP1 accumulation in hepatic stellate cells (HSCs) and inhibits HSCs overactivation. Interestingly, complete resolution of liver inflammation, significant collagen degradation, and HSCs deactivation are observed in methionine choline deficiency (MCD) and CCl4 models after tail vein injection of MUA/Y. Overall, this work reveals a macrophage-focused regulatory treatment strategy to eliminate LF progression at the source, providing a new perspective for the clinical treatment of advanced LF.
Article:https://doi.org/10.1002/advs.202306899